Avastin vs Lucentis
Thursday, 13 June 2013
The Picture Says It All.
The much anticipated results of Comparison of AMD Treatment Trial (CATT) study were out earlier this month.
Here is the summary….
Results
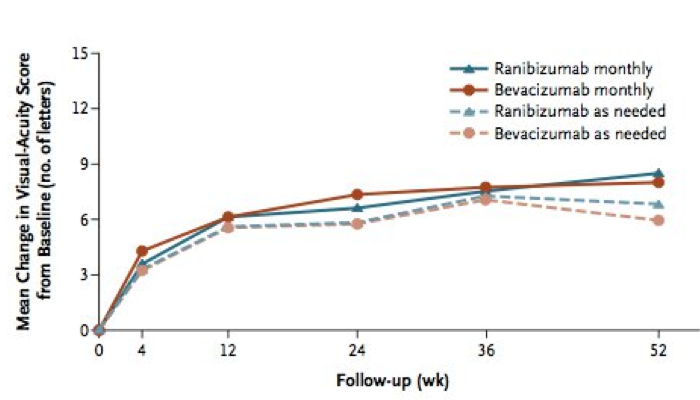
Mean gain in visual acuity at 1 year
1. Lucentis given monthly 8.5 letters
2. Avastin given monthly 8.0 letters
3. Lucentis PRN 6.8 letters
4. Avastin PRN 5.9 letters
ETDRS vision chart
When considering 5 letters difference as a clinically meaningful effect, there was no statistical difference between the groups. Note that in previous trials for AMD a meaningful effect was defined as 3 or more lines on EDTRS (15 letters). Which means that this study was powered to pick up even a small difference between the efficacy of Lucentis and Avastin.
Safety
The difference between serious adverse events (= hospitalisation for any cause) was higher in Avastin compared to Lucentis group (26% vs 19%). The study was not powered to pick up rare but serious side effects. Trials with larger sample sizes are needed to prove the difference, if there is one. The differences in this study are probably a chance finding because:
1. There was an imbalance in baseline health between Avastin and Lucentis patients. More of the former had diabetes, hypertension, congestive heart failure and other medical conditions.
2. Excess events were broadly distributed across disease categories (eg. pneumonia, surgical procedures) not identified in previous studies as areas of concern when Avastin was used at 500 time higher dosage in cancer trials
3. More side effects for both drugs when they were used less (in PRN groups).
There was no difference in the incidence of heart attacks, hypertension, stroke etc. (areas of concern in previous studies) between the two drugs.
Cost
Lucentis - $2,000 (Rs 80,000 in Pakistan)
Avastin- $50 (Rs 3,500 in Pakistan)
Future
I am open to the fact that efficacy findings for wet AMD may not transfer to patients with other conditions, such as RVO and DME, and that these drugs may behave differently for individual patients. CATT study proved that Avastin is non-inferior to Lucentis in wet AMD. The onus is now on Lucentis to prove it's superiority over Avastin in other clinical scenarios requiring VEGF inhibition.
References
1: CATT Research Group, Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011 19;364:1897-908. Free full text http://www.nejm.org/doi/pdf/10.1056/NEJMoa1102673